Beyond the Scale: GLP-1s Redefining Health Breakthroughs by 2026
 GLP-1s are ushering in a new era of systemic disease modification, impacting far more than just weight.This image is a conceptual illustration representing the broad therapeutic impact of GLP-1 receptor agonists and does not depict actual medical devices or biological processes.
GLP-1s are ushering in a new era of systemic disease modification, impacting far more than just weight.This image is a conceptual illustration representing the broad therapeutic impact of GLP-1 receptor agonists and does not depict actual medical devices or biological processes.The medical world is buzzing, and for good reason! We're witnessing what many researchers are calling a 'Metabolic Renaissance' [4]. Forget just managing symptoms; we're now in an era of systemic disease modification. At the heart of this exciting transformation are glucagon-like peptide-1 (GLP-1) receptor agonists. By 2026, these medications have moved far beyond their initial role in diabetes and weight management, becoming central to multi-organ health [1].
It's a big shift in how we see things. Obesity, for instance, isn't just a risk factor anymore; it's a chronic, systemic disease that needs long-term, structured care, often by activating complex metabolic pathways [3]. By early 2026, the wealth of high-quality data from trials in cardiovascular disease, chronic kidney disease (CKD), and metabolic dysfunction-associated steatohepatitis (MASH) has solidified GLP-1 receptor agonists as crucial tools in modern clinical practice [2].
The journey of these therapies towards 2026 has been marked by several game-changing developments: the arrival of triple-hormone agonists offering unprecedented weight loss, the convenience of oral formulations boosting patient adherence, and new national health policies making these treatments more accessible by reducing financial hurdles [3]. Of course, challenges like long-term maintenance, preserving muscle mass, and neurological efficacy still exist. But this 'metabolic renaissance' is fundamentally reshaping how healthcare systems tackle the biggest causes of death worldwide [4].
The Evolution of Pharmacological Mechanisms and Triple-Hormone Synergy
The leap from first-generation GLP-1 agonists to the multi-targeted agonists nearing regulatory approval in 2026 is nothing short of extraordinary in endocrinology and metabolic science. While earlier medications like exenatide and liraglutide proved the class's safety and effectiveness, today's therapeutic landscape is dominated by co-agonists and tri-agonists that harness the combined, powerful effects of multiple gut hormones [1].
The Synergistic Mechanism of Triple Agonists
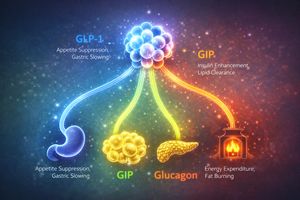
Perhaps the most significant pharmacological breakthrough by 2026 is retatrutide, a triple-receptor agonist that targets GLP-1, glucose-dependent insulinotropic polypeptide (GIP), and glucagon (GCG) receptors [10]. Unlike single-therapy approaches that primarily curb appetite via GLP-1 pathways, retatrutide activates three distinct yet complementary metabolic routes [10].
- The GLP-1 component: Works to reduce appetite and slow down gastric emptying.
- The GIP component: Enhances insulin secretion and improves the health of adipose (fat) tissue by helping clear lipids.
- The unique glucagon component: Boosts energy expenditure by promoting fat burning and thermogenesis (heat production) [10].
Clinical data through 2025 has shown that this triple activation leads to weight loss in the highest efficacy range ever observed for non-surgical interventions. In Phase 2 trials, retatrutide, at its highest dose (12 mg), achieved a remarkable 24.2% mean weight reduction over 48 weeks, with no plateau in weight loss by the trial's end [12]. This suggests that by 2026, triple agonists could offer a genuine alternative to bariatric surgery for patients with severe obesity and complex metabolic issues [10].
 Triple-receptor agonists like Retatrutide combine the power of multiple gut hormones for enhanced metabolic benefits.This image is a conceptual illustration of the pharmacological mechanisms and should not be interpreted as a literal representation of biological processes.
Triple-receptor agonists like Retatrutide combine the power of multiple gut hormones for enhanced metabolic benefits.This image is a conceptual illustration of the pharmacological mechanisms and should not be interpreted as a literal representation of biological processes.The Oral Formulation Breakthrough: A Pill, Not a Prick
Another major narrative for 2026 is the 'oral revolution' in GLP-1 therapy. For years, the need for subcutaneous (under-the-skin) injections was a significant hurdle to widespread adoption, especially in basic primary care settings [13]. To overcome this, pharmaceutical giants Novo Nordisk and Eli Lilly have pushed forward with oral versions of semaglutide and orforglipron, respectively [13].
High-dose oral semaglutide (Rybelsus at 25 mg and 50 mg) is expected to receive approval for weight loss and secondary cardiovascular risk reduction by early 2026, offering an average weight loss of 13.6% over 64 weeks [13]. This is a game-changer for patient convenience!
Orforglipron represents a further manufacturing innovation: it's a non-peptide small molecule agonist [9]. Unlike traditional peptide GLP-1s, which require biological manufacturing and cold-chain storage, orforglipron can be chemically synthesized and stored at room temperature [9]. This is a critical development for the 2026 global health agenda, as it makes distribution much easier in low- and middle-income countries where refrigeration infrastructure might be limited [14].
Cardiorenal Protection: A Weight-Independent Paradigm
One of the most impactful shifts in clinical guidelines leading into 2026 is the understanding that GLP-1 receptor agonists provide substantial cardiovascular and renal (kidney) protection, largely independent of the actual amount of weight lost [16]. This realization has transformed medications like semaglutide and tirzepatide from mere 'weight-loss drugs' into 'disease-modifying treatments' for high-risk populations [16].
The SELECT Trial and Myocardial Inflammation
An analysis of the Phase 3 SELECT trial, involving 17,604 non-diabetic participants with established cardiovascular disease, showed a 20% reduction in major adverse cardiovascular events (MACE) [1]. Crucially, sub-analyses published in late 2025 revealed that while about one-third (33%) of this benefit was due to reductions in waist circumference (a good indicator of visceral fat), the remaining two-thirds (67%) was likely driven by direct anti-inflammatory and vascular effects [16]. That's a huge insight!
The mechanisms are thought to involve a rapid decrease in systemic inflammation and epicardial adipose tissue (EAT) thickness—that's the metabolically active fat around the heart that releases pro-inflammatory cytokines (signaling molecules) [16]. By 2026, cardiology clinics are increasingly prescribing these medications to patients with heart failure with preserved ejection fraction (HFpEF) and high cardiovascular risk, regardless of their starting Body Mass Index (BMI) [16].
Renal Health and the FLOW Trial Results
The profound impact of GLP-1s on chronic kidney disease (CKD) reached a major milestone with the results of the FLOW trial. Semaglutide 1.0 mg demonstrated a 24% reduction in the risk of kidney-related events and cardiovascular death in patients with type 2 diabetes and CKD [6]. The trial showed that semaglutide's nephroprotective (kidney-protecting) effects—like reducing albuminuria (protein in urine) and lessening oxidative stress—were consistent across all severities of kidney function [6]. This data has firmly established semaglutide as a first-line treatment for diabetic kidney disease, providing a critical tool to prevent progression to dialysis [6].
Hepatology Breakthroughs: The MASH Milestone
One of the most highly anticipated health breakthroughs of 2026 is the widespread clinical adoption of GLP-1s for Metabolic dysfunction-associated steatohepatitis (MASH). This progressive liver disease affects nearly 15 million American adults [5]. In August 2025, the FDA granted accelerated approval to semaglutide (Wegovy) for treating MASH in adults with moderate-to-advanced liver fibrosis [5]. This is a big deal for liver health!
Histological Resolution and Fibrosis Regression
The ESSENCE trial provided the crucial evidence for this landmark approval. Interim 72-week results showed that 63% of participants receiving Wegovy achieved MASH resolution without worsening of liver scarring, compared to just 34% in the placebo group [17]. Even more significantly, 37% of patients on Wegovy experienced a reduction in liver fibrosis (scarring) by at least one stage, representing one of the few pharmacological successes in reversing established liver scarring [5].
By 2026, hepatologists (liver specialists) are integrating GLP-1 therapy into the standard of care for metabolic liver disease. Semaglutide's 'whole-body' metabolic benefits—improving insulin sensitivity, reducing hepatic (liver) fat accumulation, and dampening systemic inflammation—make it a powerful tool in preventing cirrhosis and the eventual need for liver transplantation [5].
Neurological Outcomes: Disappointment and Future Directions
While GLP-1s have soared in many areas, their journey in neurodegenerative diseases like Alzheimer's and Parkinson's has faced significant hurdles. Despite promising preclinical and epidemiological data suggesting neuroprotective potential, large-scale Phase 3 trials concluding in late 2025 have reported mixed results [18].
Alzheimer’s Disease and the EVOKE Trials
The results from the EVOKE and EVOKE+ trials, released in December 2025, revealed that oral semaglutide did not significantly slow the progression of cognitive decline in patients with early-stage symptomatic Alzheimer's over a two-year period [18]. While the drug did show improvement in certain biomarkers (measurable indicators of a biological state), such as markers of brain inflammation and nerve cell damage, this didn't translate into a clinically meaningful delay in symptom progression [19].
Researchers suggest that by the time Alzheimer's symptoms appear, the deficit in insulin signaling and the accumulation of toxic proteins might be too advanced for current GLP-1 dosages to overcome [18]. However, the 2026 research agenda is shifting towards even earlier intervention in preclinical populations and investigating whether dual or triple agonists can reach the necessary brain concentrations for a disease-modifying effect [20].
Parkinson’s Disease and the Exenatide-PD3 Failure
Similarly, the results of the Exenatide-PD3 trial, published in February 2025, showed that exenatide did not slow the progression of motor symptoms in people with moderate Parkinson's disease [21]. This was a significant disappointment, especially after promising Phase 2 results with lixisenatide had suggested that targeting cerebral insulin resistance could preserve motor function [21].
One theory for this setback is that the concentration of exenatide reaching the central nervous system was simply too low [22]. Consequently, the outlook for 2026 in neurology is focused on next-generation delivery methods—like intranasal administration or nanoparticles—that could more effectively bypass the blood-brain barrier [20].
Reproductive and Respiratory Health: New Therapeutic Horizons
As we gaze towards 2026, two specialized areas of medicine are experiencing a profound transformation thanks to GLP-1 and GLP-1/GIP agonists: reproductive health and sleep medicine.
Polycystic Ovary Syndrome (PCOS) and Fertility
PCOS is a complex hormonal disorder characterized by insulin resistance and an excess of androgens (male hormones), affecting millions of women of reproductive age. Real-world data through 2025 indicates a staggering more than 7-fold increase in GLP-1 and GLP-1/GIP prescriptions for women diagnosed with PCOS [23].
While not yet specifically FDA-approved for PCOS, clinicians are using these medications 'off-label' to address the metabolic comorbidities (co-occurring conditions) of the syndrome [15]. Pilot studies have shown a strong correlation between GLP-1-induced weight loss and metabolic stabilization with improved menstrual regularity and higher pregnancy rates [15]. Early research in 2026 is also exploring whether GLP-1s can directly reduce ovarian inflammation and lower circulating androgen levels through mechanisms unrelated to weight loss [15]. A truly multifaceted approach!
Obstructive Sleep Apnea (OSA) and Zepbound
In December 2024, the FDA approved tirzepatide (Zepbound) for the treatment of moderate-to-severe OSA in adults with obesity [24]. The SURMOUNT-OSA trial showed that tirzepatide led to a clinically significant reduction in the Apnea-Hypopnea Index (AHI), with participants experiencing nearly 30 fewer breathing-cessation events per hour [25].
By 2026, GLP-1 and GLP-1/GIP therapies are recognized as essential tools in sleep medicine. They don't just tackle the physical blockage caused by upper-airway adiposity (fat); they also provide systemic anti-inflammatory benefits that improve overall respiratory health and quality of life [25]. It's truly a breath of fresh air for those suffering from OSA!
 Tirzepatide (Zepbound) offers a significant breakthrough in treating moderate-to-severe obstructive sleep apnea, improving both breathing and quality of life.This image is a conceptual representation of the potential benefits of GLP-1 agonists for obstructive sleep apnea and is not a medical endorsement or guarantee of results for individuals.
Tirzepatide (Zepbound) offers a significant breakthrough in treating moderate-to-severe obstructive sleep apnea, improving both breathing and quality of life.This image is a conceptual representation of the potential benefits of GLP-1 agonists for obstructive sleep apnea and is not a medical endorsement or guarantee of results for individuals.Behavioral Health: Addressing Substance Use Disorder
A growing body of evidence suggests that GLP-1 receptor agonists might just revolutionize addiction treatment by modulating the brain's reward system. By 2026, the results of several major clinical trials investigating GLP-1s for Alcohol Use Disorder (AUD) and nicotine addiction are expected to be made public [26].
The Reward-Related Pathway Mechanism
GLP-1 receptors are found in the brain's 'reward' centers, such as the nucleus accumbens and the ventral tegmental area [11]. Activating these receptors appears to dampen the dopamine 'spikes' associated with highly rewarding stimuli, effectively suppressing 'hedonic' consumption—eating or drinking for pleasure rather than survival [25].
Preliminary studies have already shown that patients taking semaglutide or tirzepatide for weight loss frequently report a spontaneous reduction in cravings for alcohol and tobacco [26]. Case reports and remote clinical studies have already indicated significant reductions in daily alcohol consumption and symptoms of AUD in patients using these medications [26]. In 2026, this research is expanding to include opioid use disorder and cannabis use disorder, potentially offering the first pharmacotherapy (drug treatment) capable of addressing polysubstance use [26]. A truly promising avenue for complex health challenges.
Economic Impact and National Health Policy in 2026
The soaring demand for GLP-1 therapies has created both a financial challenge and a policy opportunity for health systems. By 2026, the United States and other major economies are putting new frameworks in place to manage costs and broaden the accessibility of these 'blockbuster' drugs [3].
The TrumpRx Agreement and Medicare Pricing
In late 2025, the U.S. administration announced landmark agreements with Novo Nordisk and Eli Lilly to significantly lower the price of GLP-1 weight-loss medications for eligible patients [3]. This initiative, known as 'TrumpRx,' allows cash-paying and Medicare beneficiaries to access branded weight-loss drugs for as low as $245 per month, a substantial reduction from previous list prices exceeding $1,000 [3]. This is a massive win for affordability!
Starting in April 2026, the Centers for Medicare & Medicaid Services (CMS) are launching a pilot program to cover GLP-1s for weight loss within Medicare Part D [7]. This is expected to be followed by the BALANCE Model in 2027, which will negotiate drug pricing on behalf of state Medicaid agencies [27]. These moves signal a federal recognition of obesity as a chronic condition that warrants preventative treatment to avoid more expensive complications like heart failure and kidney transplant [27].
Long-Term Employer Savings and Workforce Health
Insurance and benefits data from 2026 are already providing evidence that the upfront cost of GLP-1 therapy can be offset by long-term savings in medical spending. A multi-year study of 192,000 GLP-1 users revealed that consistent adherence (at least 80% of the time) resulted in a 9-percentage-point reduction in medical cost growth over 30 months compared to non-users [29].
For female employees, the clinical benefits were especially pronounced, with a 47% reduction in hospitalizations for major cardiovascular events and a 50% lower incidence of ovarian cancer among GLP-1 users [29]. These compelling findings are prompting employers to shift away from strictly limiting access and towards integrated 'metabolic health' benefits that prioritize long-term adherence and lifestyle support [9].
The Body Composition Challenge: Sarcopenia and Muscle Health
While the 2026 outlook for GLP-1s is overwhelmingly positive, a critical clinical concern remains: the loss of lean muscle mass during rapid weight reduction. Data from clinical trials show that while a significant portion of the weight lost is indeed fat, as much as 25% to 40% can come from lean tissue compartments [8]. This is a point to carefully consider.
Mitigating Muscle Wasting and 'Sarcopenic Obesity'
The loss of muscle mass, known as sarcopenia, is an independent risk factor for all-cause mortality and can, paradoxically, worsen insulin resistance [8]. This has led to a new trend in metabolic medicine for 2026: the integration of 'muscle-sparing' strategies with GLP-1 therapy [30].
Research is currently exploring combinations of GLP-1s with myostatin inhibitors and other myokine-based therapies to preserve strength and physical performance [30]. Health systems are also increasingly adopting digital companions that monitor protein intake and physical activity to ensure that the weight lost is high-quality adipose tissue rather than vital functional muscle [28]. For example, in the REDEFINE 1 trial, participants on CagriSema lost 35.7% of fat mass alongside 14.4% of lean tissue mass, underscoring the physiological component of body composition change that demands proactive management [31].
Global Health and the WHO Essential Medicines List
The global significance of GLP-1 therapies was officially recognized in late 2025 by the World Health Organization (WHO), which added them to the Essential Medicines List for managing high-risk type 2 diabetes and released its first global guideline for treating obesity as a chronic disease [4]. This is a monumental step!
However, the 2026 global health challenge revolves around equity. Despite rapid increases in manufacturing—including the resolution of FDA-listed shortages in April 2025—it's projected that fewer than 10% of individuals who could benefit from GLP-1s globally will have access by 2030 [3]. The WHO is advocating for pooled procurement, tiered pricing, and voluntary licensing to ensure that the breakthroughs in cardiovascular, renal, and liver health observed in 2026 can be realized in low- and middle-income countries, where metabolic disease prevalence is rising most rapidly [4]. The goal is to make these life-changing treatments available to all who need them.
Synthesis: The 2026 Metabolic Health Standard
By 2026, the medical community's understanding of GLP-1 receptor agonists has profoundly evolved. No longer just aids for glycemic or weight management, they are now conceptualized as systemic regulators of metabolic and inflammatory health [2]. The integration of these therapies into cardiology, nephrology, and hepatology has set a new standard of care where treating the root cause of metabolic dysfunction—rather than merely the symptoms—is the primary objective [5]. We are truly on the cusp of a medical revolution.
The landscape of 2026 is defined by several pivotal transitions:
- From Injections to Ingestibles: The widespread availability of high-dose oral agonists like semaglutide and small-molecule chemicals such as orforglipron is dramatically improving adherence and lowering distribution costs [9].
- From BMI to Whole-Body Diagnostics: Guidelines from the European Association for the Study of Obesity and other major bodies are replacing BMI-centric diagnoses with staging systems that prioritize cardiovascular risk, liver health, and sleep apnea [3].
- From Patient Out-of-Pocket to National Policy: Frameworks like TrumpRx and the Medicare pilot programs are actively addressing the financial barriers that previously prevented the most at-risk populations from accessing therapy [3].
- From Weight Loss to Disease Modification: The SELECT and FLOW trial results have provided definitive proof that the benefits of GLP-1s—especially in heart and kidney protection—extend far beyond the mechanical effects of shedding pounds [6].
While challenges like sarcopenia, some neurological disappointments, and global health inequities still exist, the 'Metabolic Renaissance' of 2026 marks a true turning point in human health. For the first time, clinicians possess a therapeutic class capable of modifying the trajectory of the most prevalent and expensive chronic diseases on a global scale. The task for the remainder of the decade is to ensure these pharmacological breakthroughs are supported by robust lifestyle integration, equitable access policies, and a long-term commitment to metabolic wellness [4]. The future of health looks brighter than ever!
Disclaimer: This article addresses health-related topics for informational purposes only. It does not constitute medical advice and should not replace consultation with a licensed healthcare professional. For complete guidance, please review our full disclaimer.












